CF2025
The Cystic Fibrosis External Quality Assessment scheme 2025 is organised by the European CF Network. This network works in close collaboration with EuroGentest / ESHG and the European Molecular Genetics Quality Network (EMQN).
Registration for this scheme will be open from 01/07/2024 – 31/10/2024.
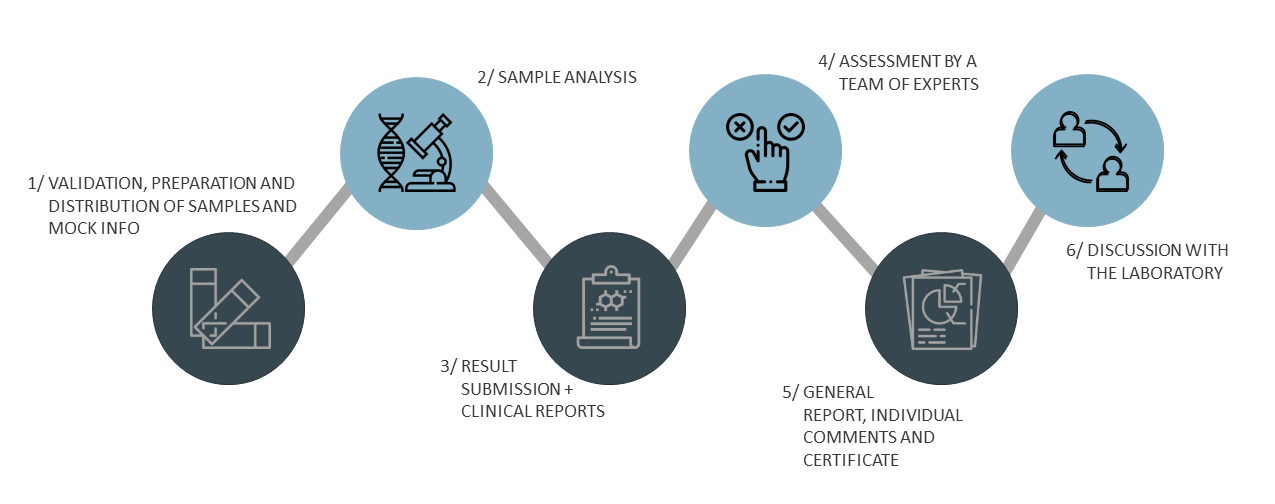
- You will receive 3 DNA samples (labeled CF25-1 to CF25-3) with clinical information (cases). New this year is that we will included also some educational paper cases. Which you are free to give feedback or not. No additional charges for this.
- The online datasheet for submitting answers will be open from January 2025 - 03/03/2025.
- Within the datasheet the laboratory should indicate which genotypes were found and which assay was used to test the EQA samples.
- In addition, the laboratory should upload written reports, including interpretation, and the raw data of the analysis.
- For the educational cases you can choose if you would like to upload your answers.
- All submitted datasheets are evaluated by a team of assessors.
- Genotype results are released in March 2025.
- A general evaluation report and individual comments will be available online from May/June 2025.
- Certificates will be given to all participating laboratories. The certificate will specify whether participation was successful or not. In addition, the certificate will mention the genotype, interpretation and clerical score.
- Invoices can be expected after closure of registration: December 2024 - January 2025. The invoice will be sent to your invoice contact person electronically from no.reply@basware.com.
CF 2025 EQA timeline
- January 2025: Distribution of the samples to the registered participants
- January - March 2025: Datasheet open; submission of genotype results and written clinical reports
- March 2025: Release of expected genotypes
- April 2025: Assessment of the results
- May/June 2025: General evaluation report and individual comments of the CF EQA scheme available online
If you have any questions, please do not hesitate to send an email to cf.network@kuleuven.be.
Read more






Head of the research unit, head EQA coordinator